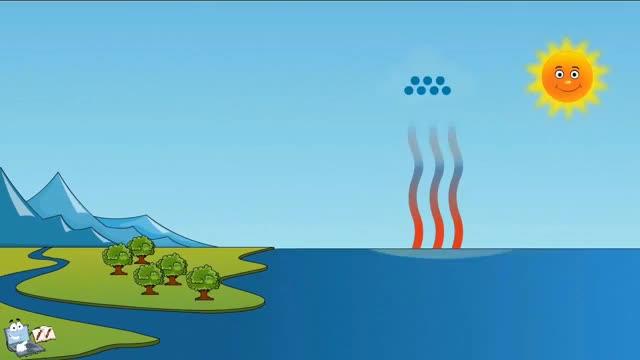
The process of water molecules escaping the surface of the Earth and entering the atmosphere is known as evaporation. Evaporation takes place as molecules of water escape from a collective body of water. This can be a puddle, a lake, a stream, or just a droplet of water.
As water molecules evaporate, they take with them some of the heat from the object from which they evaporated. This heat is stored in the water molecule, and is referred to as latent heat. The result is that the object’s temperature is lowered slightly. Consider what happens to your body on a hot day. As the temperature rises, your body begins to produce sweat. As the sweat evaporates it carries with it some of the heat from your body, causing your body to cool down.
The process of cooling down an object via evaporation is known as evaporative cooling. Many air conditioners are actually evaporative coolers, and work by taking advantage of this process.
Some important factors affecting the speed of evaporation are temperature, the amount of water vapor already in the air, and the local wind speed.
Evaporation and Temperature
Evaporation can take place at any temperature. However, increasing the temperature of a body of water also often increases evaporation. This is because as the temperature rises, the water molecules begin moving about more rapidly. This increases the odds that molecules will escape.
Warmer air also affects the speed of evaporation. Even if a body of water is cool, warm air above the water can transport some of its energy to the water molecules, allowing them to escape more rapidly.
Amount of Water Vapor in the Air
There is a limit to how much water vapor can enter the atmosphere. As more water vapor enters the atmosphere, the amount of pressure exerted by that water vapor increases. We call this pressure the vapor pressure. The higher the temperature of the atmosphere, the more vapor pressure it can withstand. When the vapor pressure maximum is reached, no more water can enter the atmosphere. At this point, we say that the atmosphere is completely saturated.
Because the maximum vapor pressure increases with temperature, warmer air can hold more water vapor before becoming saturated.
Evaporation and Local Wind Speed
Wind changes the maximum vapor pressure for the air in the atmosphere. As wind moves the air about rapidly, it causes the air to expand. This creates more room for additional water vapor. Thus, even if the maximum vapor pressure has already been reached, evaporation can continue if the wind begins to blow.