Mixture
A mixture is a combination of two or more substances that are physically combined but not chemically bonded. Keep reading to learn about the different types of mixtures, how they are made, and some examples.
In science, when two or more substances are combined physically but not chemically, this is called a mixture. For example, water and salt are separate compounds that once mixed together create seawater–a mixture. Interestingly enough, each substance retains its original properties in the process and remains its own individual substance.
Mixtures can be physically separated into individual pure substances without undergoing a chemical reaction. This is accomplished through specific methods, such as filtration and distillation.
Mixtures are two or more items combined physically, but not chemically. Some examples of mixtures include:
Cement (sand, water, gravel)
The Atmosphere (Different types of Gasses)
A Laundry Basket Filled With Dirty Clothes
Seawater (salt and water)
Trailmix
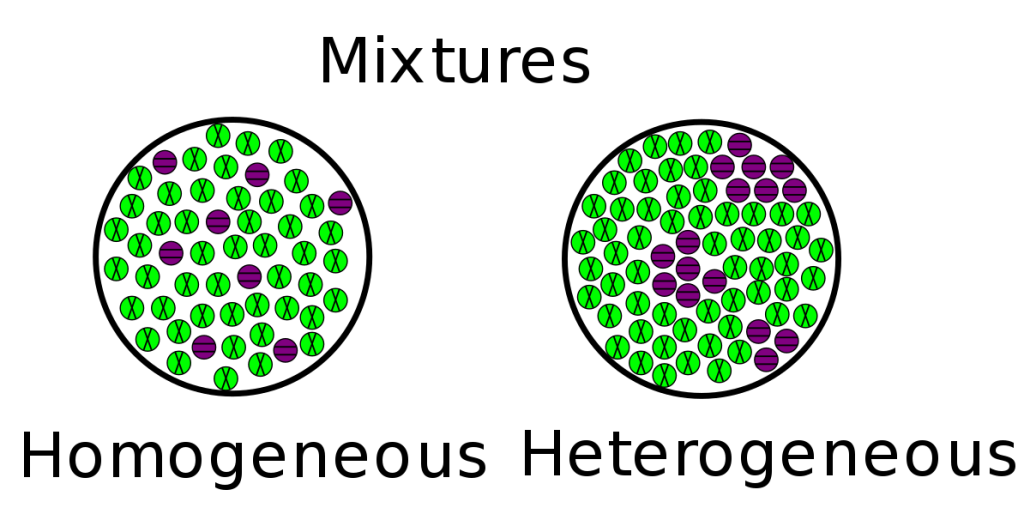
Scientists recognize two primary categories of mixtures. Which are heterogeneous and homogeneous.
Heterogeneous Mixture
Heterogeneous mixtures are sometimes confused with compounds because both have more than one kind of atom. However, they are two very different things. The minerals in a rock specimen are an excellent example of this. The atoms in a rock that do not chemically bind. They are only sitting together in the same heterogeneous mixture. These minerals forms bands or layers throughout the rocks; however, these materials can be physically separated by hand picking them out or using a table knife to scrape them apart.
In a heterogeneous mixture, the substances are not equally mixed up and they are not well distributed throughout the mixture. Thus, different areas will have more or less of some of the substances. An example of this might be a layered cake, where some parts of the cake have more frosting, and other parts have less frosting.
Homogeneous Mixture
Homogeneous mixtures have a uniform composition throughout the mixture, and the elements of the mixture cannot be separated. Homogeneous mixtures are also known as solutions.
A cup of coffee is the perfect way to start your morning. The rich flavor of the coffee grounds mix with water, and then you can add milk and sugar to create a delicious drink. Once all the ingredients are combined together, they cannot be separated. Because they are evenly spread out, they form a homogeneous mixture.
What are the features or properties of a mixture?
mixtures are created when two substances combine physically – not through a chemical reaction. Mixture properties include:
Mixtures can be separated using physical methods like filtration, freezing and distillation. Mixtures require very little energy to create compared to compounds. composition of a mixture may change over time while the composition of a compound will stay fixed. When mixed together, substances in a mixture retain their original properties while in a chemical reaction these same substances would produce new materials with different properties
Mixtures are physical combinations of different parts that have not been joined together chemically. This means the atoms in a mixture have not been changed, and each component part of the mixture still has its own independent properties.
What’s the difference between mixtures and compounds?
Mixtures are created when two or more substances are combined physically; they are not chemically bonded. Compounds, however, pure substances made up of identical molecules, occur when atoms from different chemical elements produce a chemical reaction and combine.
For example, water is created when two hydrogen atoms and one oxygen atom fuse together. The atoms have chemically combined to form water molecules. This is a new substance. However, there is no such thing as a saltwater molecule. Instead, salt water is formed when salt dissolves into water. Salt and water retain their separate characteristics. They sit together side by side, but they do not chemically bond or join.