One of the most common areas of chemistry that people interact with is using acids and bases. Acids can be found in lemons, sports drinks, pickles, and more! Bases can be found in detergents, ammonia cleaners, smoke detectors, and even baking soda.
This article will explain how acids and bases react to one-another. Then it will present some practical examples of acids and bases at home! Finally this articles will show you how to use litmus paper to test if something is an acid or base.
An acid contains hydrogen ions (H+) . A base has hydroxide ions (OH-) . When an acid comes into contact with a base they neutralize each other by making a salt and water. This reaction is known as a neutralization reaction .
For example, when you add lemon juice to baking soda an exothermic reaction occurs. The lemons have citric acid which supplies hydrogen ions. In this case, the baking soda has sodium bicarbonate which supplies hydroxide ions. The two ion types attract each other and form salt (sodium citrate) and water:
2 NaHCO3 + H+ = Na2CO3 + 2 H2O
((baking soda)) + {hydrogen ion} = {sodium bicarbonate} + {water}
Household acids are added to cleaning products to change the pH level from basic (high concentration of hydroxide ions) to acidic (higher concentration of hydrogen ions). Acidic solutions help dissolve stains easily while basic solutions add corrosiveness to dissolve tough stains. For example, ammonia is a base so it creates an alkaline solution when added to water which makes it better at dissolving tough stains like mud and blood. Bleach is an acid so it creates acidic solutions to dissolve light stains.
Acids and bases react very quickly with one-another. If you spill household cleaners or acids on yourself your skin can be burned by the reaction! This is why it is important to always wear protective gear like gloves and safety goggles when working with acids and bases. Additionally, you should always put acidic or basic solutions in separate areas of the house because their reactions can be violent .
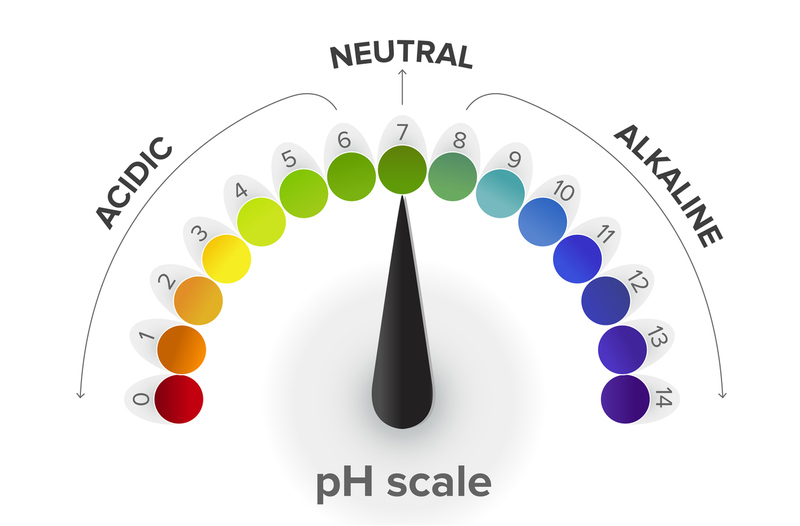
We measure Acids and bases using the PH Scale. Acids and bases have opposing PH levels. Where acids have low PH levels, and bases have high PH levels.
We can combine acids and bases together to help neutralize them. For example vinegar has a pH level of 2 making it a base. Lye has a pH level of 14 making it an acid . If you mix the two substances together the mixture would become less basic.
To test whether you have an acid or base take a small piece of wet litmus paper which you can buy at any drugstore and hold it on the surface of your substance for 20 seconds. If the strip slowly changes colors (from red to blue) then it is an acid, if the strip turns instantly blue then it is a base. You can also use this method to test for household chemicals!